Zephyrus Innovations™ announces US FDA 510(k) clearance for VaporShield™ CSTD
10 小时前
VANCOUVER, BC, Dec. 16, 2025 /PRNewswire/ -- Zephyrus Innovations (Zephyrus), a privately-owned medical device company designing and manufacturing safety syringes and Closed System Transfer Devices (CSTDs), today announces that it has received product 510(k) Marketing Clearance from the US Food and Drug Administration ("FDA") for its VaporShield ™ CSTD.
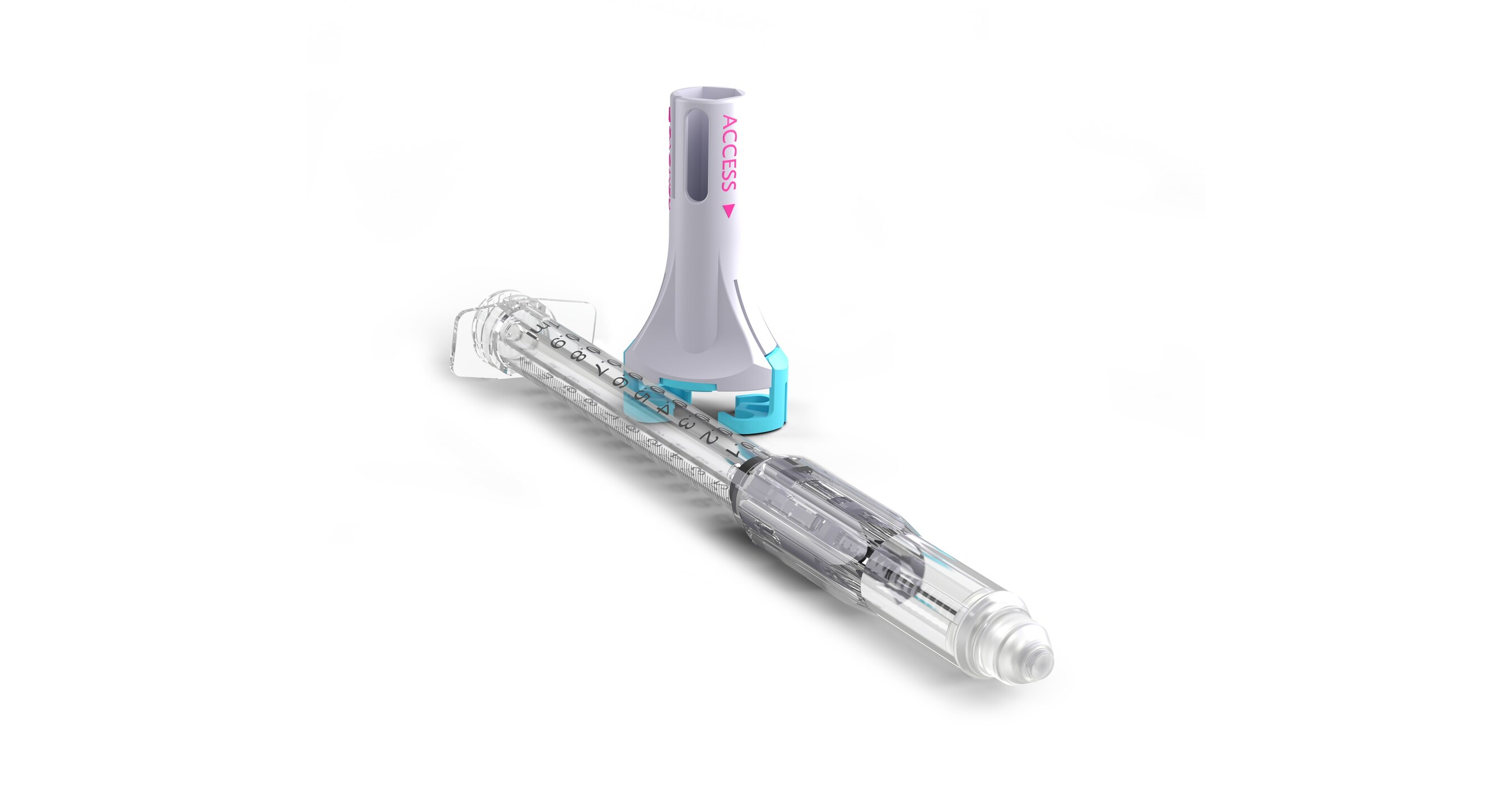
The VaporShield™ CSTD is the latest innovation in a portfolio of safety syringes being developed by Zephyrus. CSTDs are critical to protecting healthcare workers from hazardous drug exposure, which is now required in the US under USP legislation. Currently, there are no options that meet this legislation for healthcare workers who deliver subcutaneous or intramuscular injections. VaporShield provides a true closed system from drug draw to injection and through to disposal, while retaining the crucial safety syringe features of its Aeroject™ auto-retractable syringe to prevent needlestick injuries. The global CSTD market is expected to reach around $2.7 Billion by 2030, with the US accounting for 40% of sales.
Guy Reynolds, Executive Chairman of Zephyrus, said : "VaporShield is a truly game-changing device. The seriously negative health impacts caused by exposure to hazardous drugs are well documented but unfortunately, there has been no device that fully protects healthcare workers giving these drugs via subcutaneous or intramuscular administration, until now. There is now a significant opportunity to protect millions of healthcare workers around the world as they perform their day-to-day jobs."
"The development of this device and its clearance has been a remarkable team effort, drawing on the extensive experience that we have across the business. I would like to thank them, along with our shareholders, for their commitment and support to date."
About Zephyrus
Zephyrus Innovations is a privately-owned medical device company designing and manufacturing safety syringes and CSTDs that deliver medication effectively while protecting clinicians from harmful drug exposure and needlestick injuries, which can lead to infection with bloodborne pathogens and serious disease.
Media Contact: Tom Crockett, James Mainwaring, www.zephyrusinnovations.com, Email: [email protected]
Photo - https://mma.prnewswire.com/media/2845819/Zephyrus.jpgLogo - https://mma.prnewswire.com/media/2582128/Zephyrus_Logo.jpg
SOURCE Zephyrus Innovations Inc
...Read the fullstory
It's better on the More. News app
✅ It’s fast
✅ It’s easy to use
✅ It’s free